Abstract
Introduction
The BCL-2 inhibitor, venetoclax, is an effective treatment for chronic lymphocytic leukaemia (CLL). Most CLL patients (pts) treated with venetoclax respond, however in the relapsed/refractory (r/r) setting 30-50% patients progress after 2-3 years despite continuous treatment. Although the molecular drivers of relapse are becoming clearer, precisely how the pressure exerted by venetoclax is manifest at the cellular level to engender resistance remains unclear. In this study, we performed mass cytometric (CyTOF) profiling of CLL samples taken from pts during venetoclax dose escalation and employed in vivo modelling to determine the impact of short-term treatment upon survival pathways, proliferation and homing characteristics in both leukemic and normal immune cells.
Methods
We conducted serial analysis of peripheral blood (PB) samples from 20 pts with r/r CLL (median 2 lines therapy, range 1-5) at baseline and during venetoclax dose escalation (weekly increases of 20, 50, 100, 200, 400 mg) using deep profiling by CyTOF. We measured changes in 43 regulators of immune cells, cell death, proliferation, cell signalling and cancer-related pathways at the single-cell resolution. The data were normalized using CytofRUV, then analyzed using high-dimensional FlowSOM clustering, tSNE/UMAP visualization tools and conventional cytometric analysis. In order to distinguish cell intrinsic and extrinsic impacts, we assayed venetoclax pressure on immune cells with in vivo models using wild-type, Bak -/-Bax Δcd23 and Vav-BCL-2 transgenic mice and haematopoietic chimeras.
Results
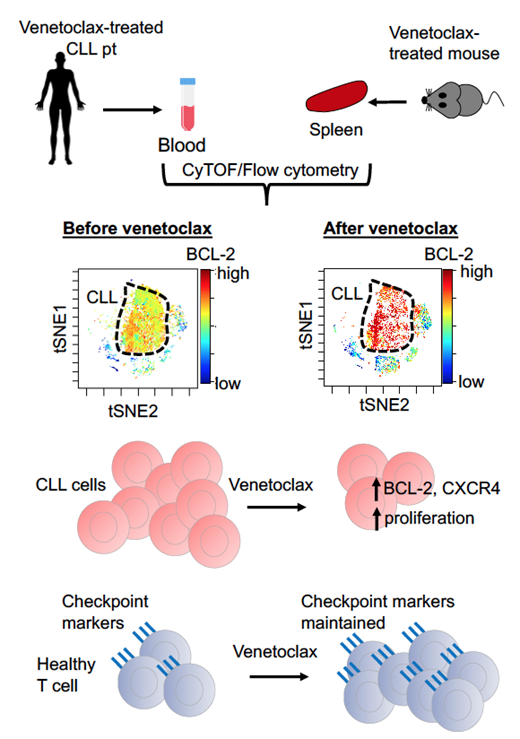
CyTOF analysis of CLL patients demonstrated inter-patient and intra-patient heterogeneity in leukemic cell populations. After six weeks of short-term venetoclax therapy, all pts showed steep decreases in CLL burden in the PB. In the remaining CLL cells, there was a striking dose-dependent increase in amounts of BCL-2, but not MCL-1 and BCL-XL protein expression. The BCL-2 +++ CLL cells remaining after venetoclax therapy were enriched for the proliferative CXCR4 high CD5 low CLL cells. As PB CLL cells are reduced upon venetoclax treatment, there were proportional increases in healthy PB T cells in CLL patients. Compared to baseline samples, all T cell subpopulations were maintained and targets of immune checkpoint inhibitors, such as PD-1, remained unchanged after venetoclax therapy.
We then modelled venetoclax responses in vivo by treating mice with 100 mg/kg venetoclax daily for 7 days and assessed the sensitivity of various immune cell subsets, their proliferation and expression of cell survival proteins. Immature lymphocytes were relatively insensitive to venetoclax, while mature naïve populations were depleted. In accord with the CLL patient data, the remaining mature B and T cells expressed very high levels of BCL-2 and were highly proliferative. Lymphocytes lacking the apoptotic effectors BAX and BAK from Bak-/-BaxΔcd23 micedid not exhibit these changes. By contrast, transgenic mice with overexpression of BCL-2 (vav-bcl-2 mice) did not rescue cells from the impacts of venetoclax. These data reveal direct and consistent impacts of venetoclax pressure in normal and leukemic cells at the single cell level.
Conclusions
Collectively, our findings reveal that short-term treatment with venetoclax exerts pressure on both CLL and healthy immune cells (Figure). Striking changes in the wiring of survival pathways in CLL cells occurred shortly after venetoclax treatment, suggesting that therapies targeting orthogonal survival pathways should be considered shortly after dose-escalation. Furthermore, the limited changes in healthy T cells opens a window for targeting these cells by adjunct immune checkpoint inhibitors to achieve deeper responses.
Teh: The Walter and Eliza Hall Institute of Medical Research: Patents & Royalties: Employee of the Walter and Eliza Hall Institute and eligilble for payments in relation to venetoclax. Tan: The Walter and Eliza Hall Institute of Medical Research: Patents & Royalties: Employee of the Walter and Eliza Hall Institute and eligilble for payments in relation to venetoclax. Trussart: The Walter and Eliza Hall Institute of Medical Research: Patents & Royalties: Employee of the Walter and Eliza Hall Institute and eligilble for payments in relation to venetoclax. Luo: The Walter and Eliza Hall Institute of Medical Research: Patents & Royalties: Employee of the Walter and Eliza Hall Institute and eligilble for payments in relation to venetoclax. Thijssen: The Walter and Eliza Hall Institute of Medical Research: Patents & Royalties: Employee of the Walter and Eliza Hall Institute and eligilble for payments in relation to venetoclax. Roberts: Janssen: Research Funding; Abbvie: Research Funding; The Walter and Eliza Hall Institute of Medical Research: Patents & Royalties: Employee of the Walter and Eliza Hall Institute and eligilble for payments in relation to venetoclax; Servier: Research Funding. Huang: The Walter and Eliza Hall Institute of Medical Research: Patents & Royalties: Employee of the Walter and Eliza Hall Institute and eligilble for payments in relation to venetoclax. Speed: The Walter and Eliza Hall Institute of Medical Research: Patents & Royalties: Employee of the Walter and Eliza Hall Institute and eligilble for payments in relation to venetoclax. Anderson: The Walter and Eliza Hall Institute: Honoraria, Patents & Royalties: Employee of the Walter and Eliza Hall Institute and eligilble for payments in relation to venetoclax, Speakers Bureau. Gray: The Walter and Eliza Hall Institute of Medical Research: Patents & Royalties: Employee of the Walter and Eliza Hall Institute and eligilble for payments in relation to venetoclax.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal